International Society for Pediatric and Adolescent Diabetes (ISPAD)
October 15-17, 2020; Virtual; Day #1 Highlights – Draft
Executive Highlights
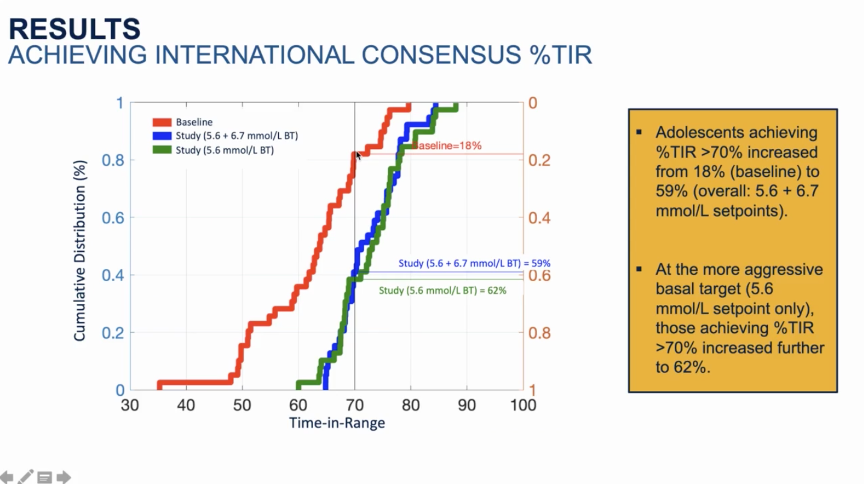
- In a morning session on AID, Tandem’s Dr. Steph Habif shared the first-ever batch of real-world data from Control-IQ in pediatrics (ages 6-14). As a reminder, Control-IQ was first cleared for this age group in June. In ~1,400 users with at least 30 days of pre- and post-Control-IQ data, Time in Range increased by an impressive 3.4 hours/day, from 53% before Control-IQ to 67% after Control-IQ. In the same session, we saw some more details from the adolescent subgroup (ages 14-21) of Medtronic MiniMed 780G’s pivotal trial. The percentage of participants achieving a >70% Time in Range goal more than tripled from 18% at baseline to 59% with MiniMed 780G. With the more aggressive 100 mg/dl set point, the percentage of participants achieving 70% Time in Range was even higher at 62%.
- On the CGM side, Children’s Hospital of Philadelphia’s Dr. Charlene Lai shared data outlining a disturbing racial disparity in rates of CGM initiation and sustained CGM use in children with type 1. During the three-year period analyzed, 54% of non-Hispanic White participants initiated CGM use while only 31% of non-Hispanic Black participants and 33% of Hispanic participants initiated CGM use; in other words, the odds of a non-Hispanic White patient to initiate CGM was 2.7 times higher than that of a non-Hispanic Black patient. Importantly, this gap held even when controlled for public and private insurance.
The first-ever virtual (and 46th overall) ISPAD conference kicked off this morning, Central Daylight Time, with participants from all over the world. See our top highlights below and our preview for a look ahead at the next two days.
Table of Contents []
-
Diabetes Technology Highlights
- 1. First Real-World Control-IQ Data from Adolescents (ages 6-13, n=1,428): +3.4 Hours/Day Time in Range, 95% Time in Closed Loop, ~1% Time <70 mg/dl
- 2. Adolescents (ages 14-21, n=39) in Medtronic Advanced Hybrid Closed Loop (MiniMed 780G) Pivotal: +2.5 Hours/Day TIR, -0.5% A1c After Three Months
- 3. Abbott Symposium: The Advantages of Flash Glucose Monitoring CGM in Pediatric Populations; Methods for Addressing Barriers to Patients’ Success in Using CGM
- 4. Retrospective Chart Review of Type 1s Shows that 54% of Non-Hispanic Children Initiated CGM from 2015-2018, Compared to 31% of Non-Hispanic Black Children; Disparities Cannot Be Explained by Insurance Status
-
Diabetes Therapy and Obesity Highlights
- 1. Professor Chantal Mathieu on the Promises and Pitfalls of Adjunctive Therapies in Type 1 Diabetes
- 2. Sanofi Symposium Addresses Barriers to Optimal Diabetes Management in Pediatric Populations: A Window of Opportunity for Second-Generation Basal Insulins, CGM, and Psychosocial Care
- 3. Comprehensive Review of Type 1 Diabetes Treatment and Prevention Progress Highlights Next Steps for Discovering Effective Therapies
- 4. Dr. Sinead Murphy on the Importance of Psychosocial Care in Adolescent Obesity
Diabetes Technology Highlights
1. First Real-World Control-IQ Data from Adolescents (ages 6-13, n=1,428): +3.4 Hours/Day Time in Range, 95% Time in Closed Loop, ~1% Time <70 mg/dl
Tandem’s Vice President of Behavioral Sciences Dr. Steph Habif gave us a first look at real-world pediatric data (ages 6-13) on Control-IQ. As a reminder, Control-IQ was first cleared for this age group in June. The data was gathered through Tandem’s t:connect software from ~1,400 users with at least 75% CGM use and at least 30 days of pre- and post-Control-IQ data. Mean age of the group was 11 years and 51% were female.
- Time in Range increased by an impressive 3.4 hours/day, from 53% before Control-IQ to 67% after Control-IQ. The improved Time in Range came entirely from reductions in time spend in hyperglycemia: time >180 mg/dl fell from 45% to 31%. At the low end, time <70 mg/dl was similar before and after Control-IQ, rising slightly from 1% to 1.2% with Control-IQ (~3 minutes/day difference). Finally, participants spent a strong 95% of time in closed loop.
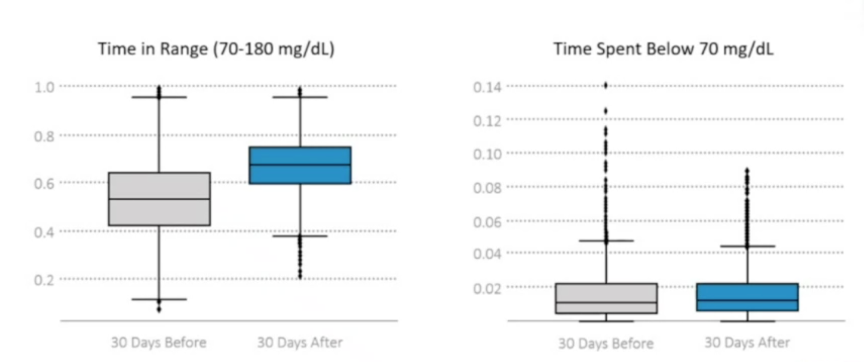
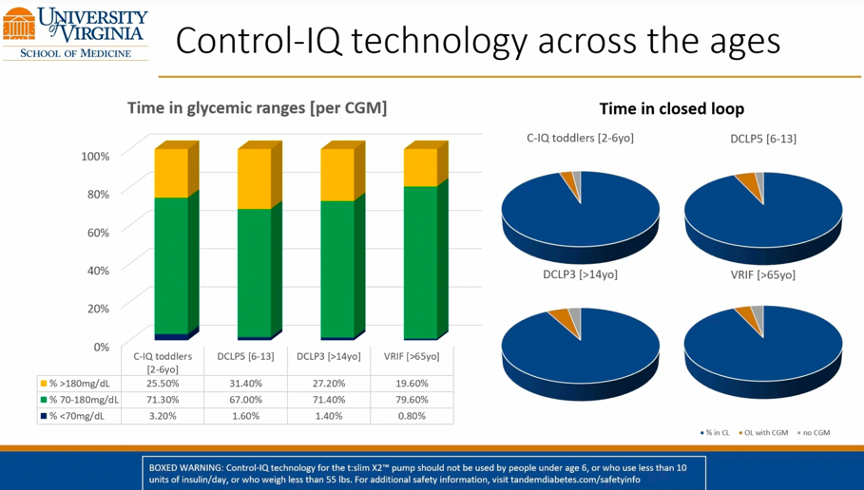
- The early real-world data is eerily similar to that of the pediatric pivotal trial, which read out at ATTD 2020 and was recently published in NEJM. In the sixteen-week pivotal trial (n=101, ages 6-13), participants also had a Time in Range of 53% at baseline, increasing to 67% at the end of the study. More broadly, the real-world pediatric data match well with real-world adult data, as well. Last month, at EASD 2020, Dr. Marc Breton (UVA) presented the latest batch of real-world adult data on Control-IQ from ~6,000 users, showing a Time in Range increase of ~2.5 hours/day just two weeks after starting Control-IQ. The slide below, taken from Dr. Breton’s presentation at EASD, shows the consistency of performance for Control-IQ across several different age groups.
2. Adolescents (ages 14-21, n=39) in Medtronic Advanced Hybrid Closed Loop (MiniMed 780G) Pivotal: +2.5 Hours/Day TIR, -0.5% A1c After Three Months
In a morning session on diabetes technology, Dr. Dorothy Shulman (University of South Florida) presented results from the 39-person adolescent subset (ages 14-21) of Medtronic’s Advanced Hybrid Closed Loop (MiniMed 780G) pivotal. Participants all had at least six months of pump experience at baseline and began with a 14-day run-in period using either sensor-augmented pump or MiniMed 670G therapy. Then, participants were randomized into either 100 mg/dl or 120 mg/dl set point groups for three months, with cross-over at 45 days.
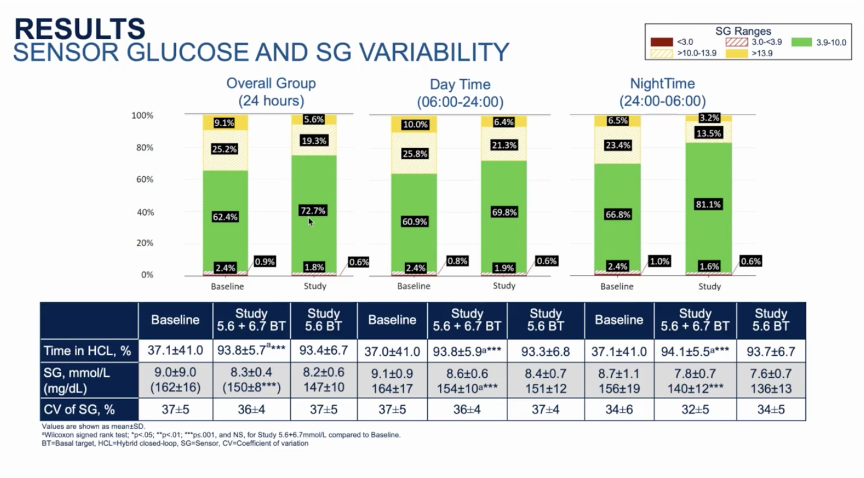
- Overall, Time in Range increased by ~2.5 hours/day from 62% at run-in to 73% during the study period. As seen with most closed loop systems, the greatest improvements were seen overnight (midnight – 6 AM) with Time in Range jumping from 67% during run-in to 81% with 780G. Daytime (6 AM – midnight) Time in Range improvements were a bit more modest, but still impressive, increasing from 61% to 70%.
- A1c decreased from 7.6% at baseline to 7.1% after three months on MiniMed 780G. Similarly, mean sensor glucose declined from 162 mg/dl during run-in to 150 mg/dl during the study period. Looking at the period with a 100 mg/dl set point only, mean glucose was even lower, at 147 mg/dl. For context, a mean glucose of 147 mg/dl corresponds to an A1c of ~6.8%.
- Throughout the study period, the mean time in closed loop was a strong 94%. The total number of closed loop exits per week was 1.2, translating to about one exit every six weeks. Of the exits, about one-third were initiated by the user, meaning the number of system-related exits was just ~0.8 per week (~one every nine days). There was just one serious adverse event during the study, a non-device related severe hypoglycemic event during run-in.
- The percentage of participants achieving a >70% Time in Range goal more than tripled from 18% at baseline to 59% with MiniMed 780G. With the more aggressive 100 mg/dl set point, the percentage of participants achieving 70% Time in Range was even higher at 62%. Similarly, the percentage of participants achieving A1c <7% more than doubled using MiniMed 780G, from 21% at baseline to 47% after three months. Importantly, all these results were achieved without significant increases in time in hypoglycemia or hypoglycemic events.
- As a reminder, results from the overall Medtronic AHCL/MiniMed 780G pivotal trial read out at ADA 2020, which included data from all 157 participants (ages 14-75). As of yesterday, we learned that MiniMed 780G has already begun shipping in Europe; on the US side, we last heard in May that the MiniMed 780G system and algorithm will launch in the US “later this fiscal year” (FY21 ends in April 2021). We’ve summarized results from the US pivotal trial in the table below, and broken out data by adolescent and adult subgroups.
|
|
Overall Group (n=157) |
Adolescents (ages 14-21; n=39) |
Adults (ages 22-75; n=118) |
|||
|
Outcome |
Baseline |
Study |
Baseline |
Study |
Baseline |
Study |
|
Time in Range |
69% |
75% |
62% |
73% |
71% |
75% |
|
A1c |
7.5% |
7% |
7.6% |
7.1% |
7.5% |
7% |
|
Time >180 mg/dl |
28% |
23% |
34% |
25% |
26% |
23% |
|
Time >250 mg/dl |
6.2% |
4.6% |
9.1% |
5.6% |
5.3% |
4.3% |
|
Time <70 mg/dl |
3.3% |
2.3% |
3.3% |
2.4% |
3.4% |
2.3% |
|
Time <54 mg/dl |
0.8% |
0.5% |
0.9% |
0.6% |
0.8% |
0.5% |
|
Mean CGM |
153 mg/dl |
148 mg/dl |
162 mg/dl |
150 mg/dl |
151 mg/dl |
147 mg/dl |
|
Time in closed loop |
33% |
95% |
37% |
94% |
32% |
95% |
Note: Time in closed loop during baseline is from some participants using the MiniMed 670G system during run-in.
3. Abbott Symposium: The Advantages of Flash Glucose Monitoring CGM in Pediatric Populations; Methods for Addressing Barriers to Patients’ Success in Using CGM
During an Abbott-sponsored symposium on Thursday morning, Dr. Nancy Elbarbary (Ain Shams University, Egypt), Dr. Fiona Campbell (Leeds Children’s Hospital, UK), and Dr. Karin Lange (Hannover Medical School, Germany) discussed the power of flash glucose monitoring in pediatric populations and offered practical advice for its implementation. Dr. Campbell encouraged organizations, hospitals, and national health systems to learn from each other’s successes, sharing her own experience applying learning from Swedish successes with CGM and glycemic control in pediatric populations to country-wide English and Welsh pediatric diabetes standard practices. She also advocated for a strong, unified national registry for pediatric technology use and glycemic outcomes so that progress can be measured, and the impact of national programs can be assessed. In her talk, Dr. Elbarbary shared the Arab Society of Pediatric Endocrinology and Diabetes experts’ six general recommendations for increasing the uptake of CGM in pediatric populations: (i) train HCPs in the use and interpretation of CGM data; (ii) collaborate with pharmaceutical partners to provide quality training; (iii) standardize metrics and reporting across different CGM devices; (iv) include summary pages in reports that are meant for patients and parents; (v) encourage patients to use CGM during Ramadan to monitor their glucose profile and practice safe fasting; and (vi) push for the study of application sites other than the upper arm.
- Dr. Lange closed out the session with a talk on the impact of flash glucose monitoring on the psychological burden and diabetes distress of young people with diabetes and their families. After flying through a plethora of evidence showing the positive impacts of CGM on quality of life for pediatric patients and their families (improved sleep, sense of safety, less family conflicts, reduced hypoglycemia avoidance behaviors, improved self-efficacy, higher diabetes treatment satisfaction), she moved into a fascinating discussion on the barriers that hinder children’s success in using CGM, saying, “CGM is a tool that cannot do anything by itself. It depends on what you do with it.” She explained the various factors which hinder children’s successes with CGM including cost, wear-related issues, diabetes distress, dislike for having a device on their body, difficulty interpreting the data, learned helplessness, unrealistic expectations, family conflicts, data gaps due to loss signals, and adhesive problems and discomfort. While some of these barriers require direct and personalized attention, Dr. Lange offered several general recommendations: (i) HCPs need to have a positive attitude toward and a strong understanding of the technology themselves; (ii) offer structured family education and CGM coaching; (iii) set realistic targets; (iv) help patients accept glucose fluctuations without feelings of guilt or fear; (v) educate patients on the lag time between blood and tissue glucose measurements, as well as on trend arrows; and (vi) communicate with empathy and draw attention to the positives and the successes.
- Dr. Elbarbary also discussed why CGM is a particularly powerful tool in the Middle East, which was an interesting context-specific discussion we appreciated. She reviewed various studies indicating that FreeStyle Libre is a particularly useful tool during fasting during Ramadan to understand hypo- and hyperglycemic events (e.g., Deeb et al. 2019, Al-Agha et al. 2017, Hassanein et al. 2019, and Bashir et al. 2018). In addition, she discussed how the high incidence of hemoglobinopathies like thalassemia, sickle cell disease, iron deficiency anemia, and G6PD deficiency in the MENA region (Middle East/North Africa) complicates the use of A1c as a measurement, meaning that CGM-based metrics like Time in Range are more reliable. Dr. Elbarbary’s point suggested two important things to us: (i) CGM-derived metrics like Time in Range are likely to be more personally meaningful than lab A1c; and (ii) it’s important for providers to avoid blindly using A1c-driven treatment algorithms to provide care for all their patients, particularly minorities.
4. Retrospective Chart Review of Type 1s Shows that 54% of Non-Hispanic Children Initiated CGM from 2015-2018, Compared to 31% of Non-Hispanic Black Children; Disparities Cannot Be Explained by Insurance Status
Dr. Charlene Lai (Children’s Hospital of Philadelphia) presented results from a retrospective chart review showing massive racial disparities in rates of CGM initiation and sustained CGM use in children with type 1. The study gathered data between 2015-2018 from 1,509 children in Pennsylvania with type 1 who were not using CGM and were <17 years old on January 1, 2015. Of the 1,509 participants, 279 were non-Hispanic Black (19%), 125 were Hispanic (8%), and 1,105 were non-Hispanic white (73%). Pennsylvania residency was required for inclusion in the study, ensuring that insurance coverage would not be a barrier for any participant; Pennsylvania state Medicaid coverage offers CGM use to all pediatric type 1s.
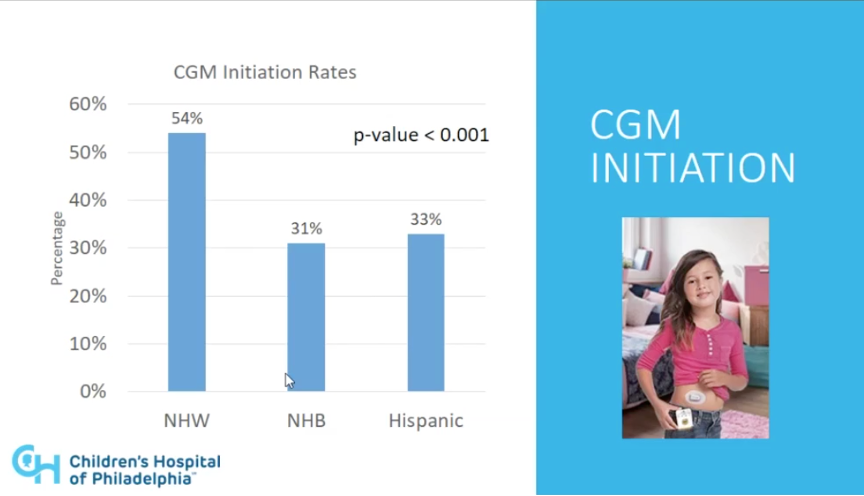
- Overall, 48% of participants began using CGM during the three-year study period. We certainly would have hoped to have seen no correlation by race. However, and although there were small numbers in the Hispanic group in particular, overall there were stark differences when segmented by race/ethnicity: 54% of non-Hispanic white participants initiated CGM use, an unusually high percentage compared to national figures as we understand it (we are checking this now), while only 31% of non-Hispanic Black participants and 33% of Hispanic participants initiated CGM use (p<0.001>. It is certainly troubling that the odds of a non-Hispanic white patient to initiate CGM was nearly 3.0 times higher than that of a non-Hispanic Black patient. Notably, this disparity persisted when segmented by insurance type, which was used as a proxy for socioeconomic status (SES). Within those who had commercial insurance, non-Hispanic White patients still had 2.3 times higher odds than non-Hispanic Black patients to start CGM. Within the government insurance group, non-Hispanic White patients had two times higher odds than non-Hispanic black patients.
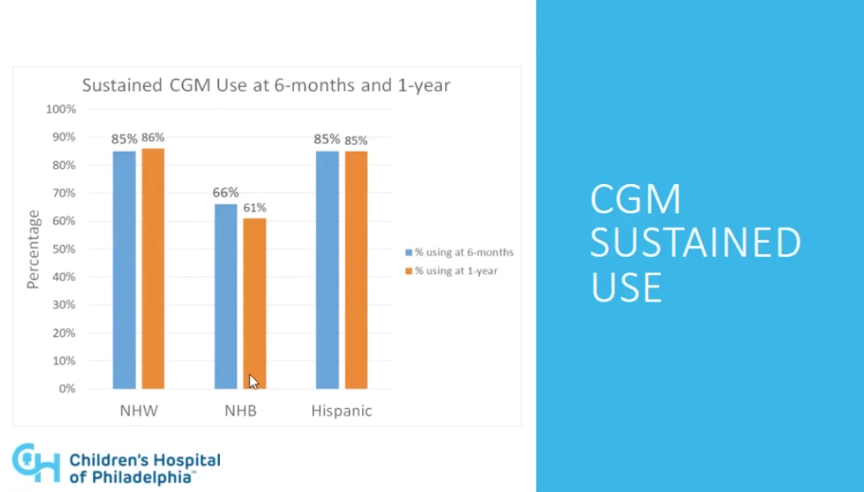
- There were also meaningful disparities in sustained use of CGM. In this case, both the non-Hispanic White and Hispanic groups achieved ~85% sustained use of CGM at six months and one year whereas non-Hispanic Black participants achieved only 66% sustainment at six months and 61% at one year. Overall, non-Hispanic White participants had 4.1 times higher odds than their non-Hispanic Black counterparts to still be using CGM after a year. Again, these results were consistent when stratified by insurance type.
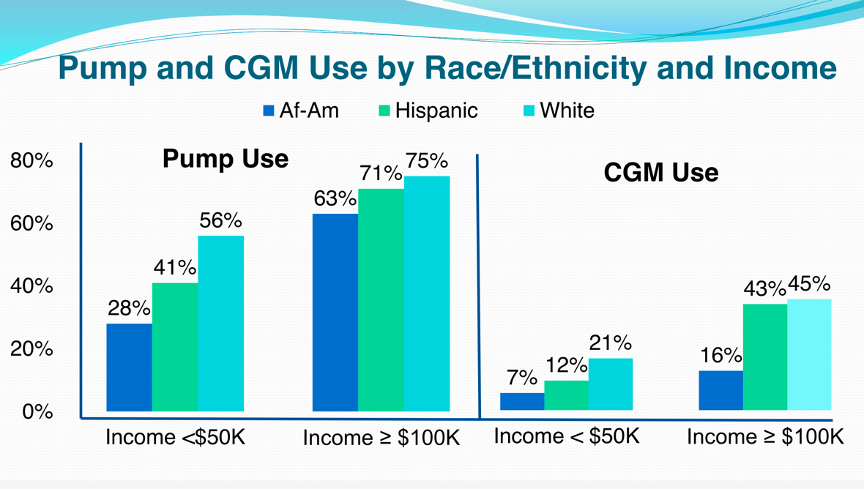
- We applaud the drive toward stratifying use and persistence and satisfaction by race, and hope more of this research is funded. The results from Pennsylvania add onto existing and growing evidence that racial disparities in diabetes technology use cannot be solely attributed to financial disparities. Data from the T1D Exchange showed that the rate of CGM use in white children was triple that of African American children across all income groups. Further highlighting this point, CGM use in white patients from families making <$50,000/year was higher than that of black patients from families making ≥$100,000/year (21% vs. 16%). Clearly, more race-specific research needs to be done in the area to help address the gap. Provider biases might be one place to start: a 2015 study in Pediatrics found that providers waited one year longer to initiate pump therapy after diabetes diagnosis in black children than white children.
Slide taken from T1D Exchange data.
Diabetes Therapy and Obesity Highlights
1. Professor Chantal Mathieu on the Promises and Pitfalls of Adjunctive Therapies in Type 1 Diabetes
Professor Chantal Mathieu (KU Leuven) discussed the benefits and drawbacks of nasal glucagon, GLP-1 receptor agonists, and SGLT-2 inhibitors in type 1 diabetes. While nasal glucagon was presented as a possible treatment option for severe hypoglycemia in pediatric type 1 populations, Professor Mathieu underscored the difficulties and often insufficient evidence to support GLP-1 receptor agonist and SGLT-2 inhibitor use in adult patients, not to mention children and adolescents. Conflicting evidence has yet to determine if GLP-1 receptor agonists increase risk of hypoglycemia in type 1, while SGLT-2 inhibitors have been repeatedly shown to increase risk of diabetic ketoacidosis (DKA). See below for Prof. Mathieu’s detailed takes on each novel treatment option.
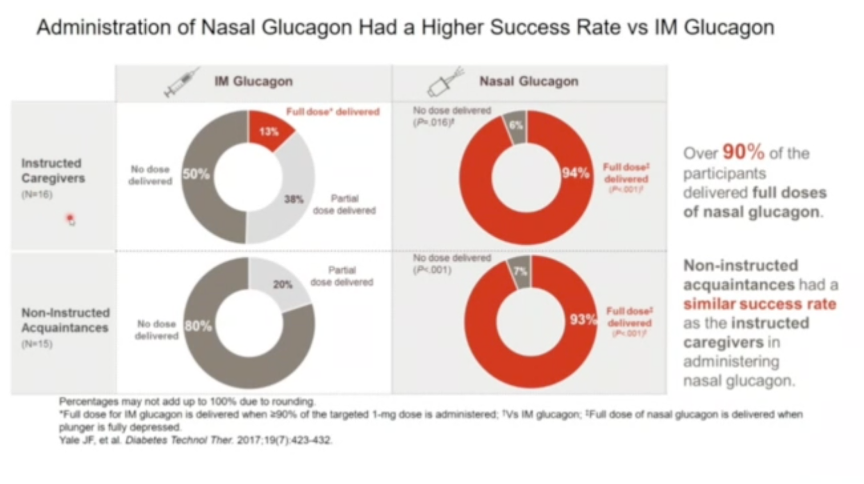
- Professor Mathieu called nasal glucagon an “interesting addition for the treatment of severe hypoglycemia” in discussing the results of four pediatric studies included in the type 1 diabetes development program: (i) a pivotal pharmacokinetic/ pharmacodynamic (PK/PD), safety, and efficacy study; (ii) a real-world use study; (iii) a simulated use study; and (iv) a supporting study looking at the effects of nasal congestion and decongestants on PK/PD. Collectively, these studies showed that nasal glucagon delivers nearly equivalent PK/PD to that of traditional intramuscular (IM) glucagon (regardless of nasal congestion and/or use of decongestants), allows for successful and rapid dosing (<2 minutes in 100% of cases), and is associated with caregiver satisfaction on the drug’s ease of administration and efficacy. In the simulated use study, caregivers also showed significantly greater success in administering nasal glucagon versus IM glucagon under “real-world” conditions (e.g. loud sounds and other stressors) to a mannequin. Even with instructions on how to use the different devices, only 13% of participants were able to deliver the full dose of IM glucagon under stressful conditions, compared to 94% when given the nasal glucagon. These data underscore the ease of use for nasal glucagon as compared to IM glucagon, which requires reconstitution before use and extensive training and attention on the part of the caregiver. At the same time, nasal glucagon use was also shown to be significantly associated with nasal discomfort, in addition to headaches, nausea, and vomiting – the latter three side effects, however, are also associated with IM glucagon.
- Prof. Mathieu also presented the results of the ADJUNT program investigating three doses of GLP-1 receptor agonist liraglutide in type 1 diabetes. ADJUCNT ONE employed a target-to-treat insulin regimen, while ADJUNCT TWO employed an insulin-capped design. While liraglutide delivered largely dose-dependent reductions in A1c, insulin requirements, and fasting body weight in both trials, ADJUNCT ONE reported a trend towards increased hypoglycemia rates in the treatment arm. As Prof. Mathieu showed in a new analysis, however, the risk of hypoglycemia was increased only in those groups without measurable C-peptide; if C-peptide was above a certain threshold for detection, liraglutide conferred no excess risk of hypoglycemia. Further investigator-initiated studies have confirmed these results, as well as no increased risk of DKA. These findings highlight the potential of GLP-1 receptor agonists in type 1 diabetes, although more research is certainly needed to confirm safety.
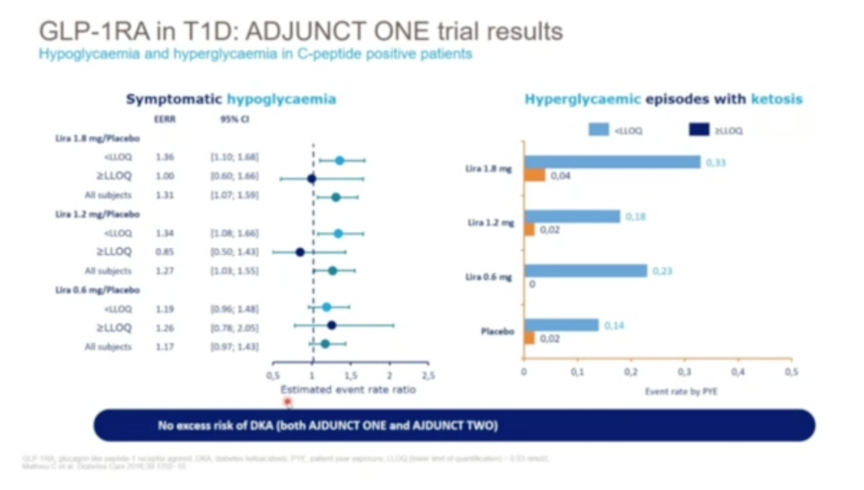
- While the SGLT-2 inhibitors dapagliflozin and sotagliflozin have been approved at low doses for type 1 diabetes in the EU (with BMI cutoffs), Prof. Mathieu exclaimed that “education, education, education” was needed for responsible use – both on the part of the patient and provider. As shown in DEPICT-1 and DEPICT-2, among other studies, while SGLT-2 inhibitors confer impressive reductions in body weight, A1c, blood pressure, and even urine albumin-to-creatinine ratio (UACR) in type 1 patients, they significantly increase risk of DKA. As a result, Prof. Mathieu recommends SGLT-2 inhibitors to type 1 diabetes patients only if they are adults, have an A1c below 9 or 10%, receive enough insulin of >20 U/day, and match the BMI cutoff set by EMA (>27 kg/m2). Even then, careful monitoring of diet, dehydration, ketones, and other factors is frequently required.
2. Sanofi Symposium Addresses Barriers to Optimal Diabetes Management in Pediatric Populations: A Window of Opportunity for Second-Generation Basal Insulins, CGM, and Psychosocial Care
In a Sanofi-sponsored symposium, Professors Thomas Danne (Hannover Medical School Germany), Tadej Battelino (University of Ljubljana, Slovenia), and Katherine Barnard-Kelly (Bournemouth University, UK) reviewed options for reducing the burden of type 1 diabetes care in children and adolescent populations. Dr. Danne highlighted the potential of second-generation basal insulins for improving outcomes in pediatric patients, while Dr. Battelino advocated for the use CGM and time in range metrics. Dr. Barnard-Kelly then put these treatment strategies in perspective, calling to attention the psychological burden of diabetes in children and offering actionable guidance for incorporating management strategies in a personalized manner.
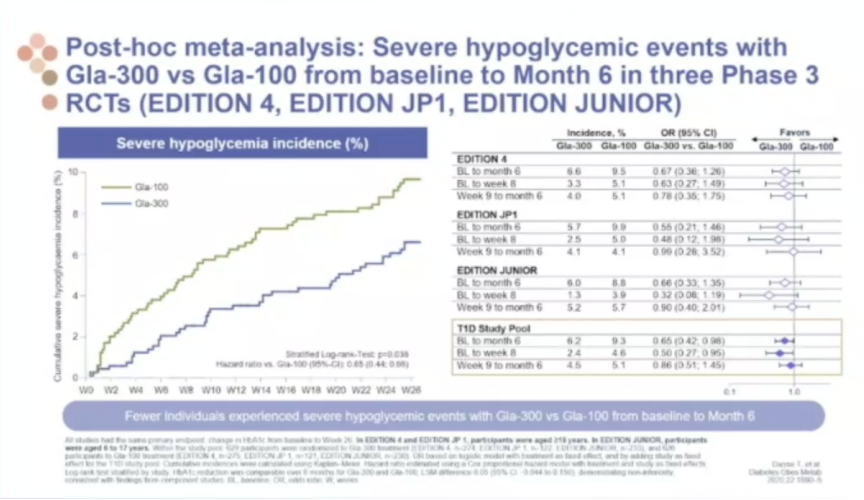
- Dr. Thomas Danne presented the results of EDITION JUNIOR and BEGIN YOUNG, two randomized-controlled trials evaluating second-generation basal insulins in pediatric patients with type 1 diabetes. These results were highlighted by Dr. Danne and others at a recent Sanofi symposium at EASD 2020, and full results of EDITION JUNIOR were released at last year’s ISPAD 2019. In type 1 patients ages 1-17, BEGIN YOUNG reported non-inferiority for Tresiba (insulin degludec) versus Levemir (insulin determir) on (i) A1c reduction from baseline to 26 weeks and (ii) rates of confirmed hypoglycemia. Tresiba, however, showed superiority over Levemir for reducing the incidence of hyperglycemia with ketosis. EDITION JUNIOR reported similar safety and efficacy data when comparing Toujeo (insulin glargine U300) to Lantus (insulin glargine U100). In type 1 patients ages 6-17, Toujeo showed non-inferiority to Lantus on 26-week A1c reductions and rates of hypoglycemia, while delivering numerically lower rates of hyperglycemia and ketosis (6% versus 9%, respectively). As Dr. Danne emphasized, these results highlight the promise of second-generation basal insulins (Tresiba and Toujeo) for pediatric populations with type 1 diabetes, particularly those “chaotic adolescents” with unpredictable eating schedules and high risk of diabetic ketoacidosis (DKA).
- Although not demonstrated in EDITION JUNIOR, Toujeo may be superior to Lantus for reducing the rate of severe hypoglycemic events. A post-hoc meta-analysis of EDITION JUNIOR, EDITION 4, and EDITION JP1 revealed just this trend, with hypoglycemia incidence of Toujeo falling below that of Lantus as early as 6 weeks and maintaining significance at 6 months.
- Professor Tadej Battelino (University of Ljubljana, Slovenia) touted the use of time in range (TIR) metrics for improving glycemic control and reducing risk of microvascular complications in pediatric populations with type 1. However, he encouraged the use of a “stepwise approach” for helping children and adolescents achieve their goals, stressing that even incremental increases in TIR – as low as 5% – should be viewed as clinically meaningful. Psychologist Dr. Katherine Barnard-Kelly (Bournemouth University, UK) took a similar perspective in her presentation, emphasizing how overwhelming and burdensome type 1 diabetes management can be for pediatric populations. Technology, as Dr. Barnard-Kelly explained, should not require so much attention as to pose an additional burden, but should be weighed with consideration of the patient’s goals and circumstances. Further, Dr. Barnard-Kelly advocated that mental health issues should be treated with “same energy and priority” as those related to the physical aspects of the condition. Through an “integrated care approach,” engaging HCPs and patients alike on the psychological, socioeconomic, and physical aspects of diabetes management, we can minimize the burdens to optimal care.
3. Comprehensive Review of Type 1 Diabetes Treatment and Prevention Progress Highlights Next Steps for Discovering Effective Therapies
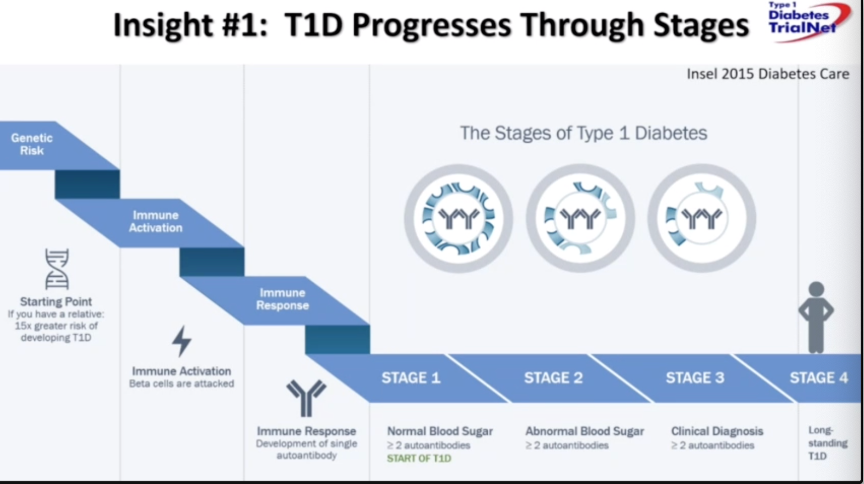
Drs. Linda DiMeglio (Indiana University School of Medicine) and Michael Haller (University of Florida) provided a critical analysis of the history of research in type 1 diabetes treatment and prevention, with focus on future next steps. Dr. DiMeglio began the session with a discussion of therapies from the discovery of insulin in 1922 to the plethora of smart devices, like AID and CGM, that promote better diabetes management. We’ve gained four main insights throughout this process of discovery: (i) type 1 diabetes progresses through stages (outlined below); (ii) we’re at the cusp of predicting who will progress from stage one to stage three; (iii) beta cell function can be preserved in stage three; and (iv) new therapies can prevent the progression from stage two to stage three. While these insights have led to the discovery of effective therapies like teplizumab, alefacept, and golimumab, areas for improvement remain. Dr. DiMeglio highlighted three main shifts to embrace for progress: embrace type 1 as a diverse syndrome, embrace change overtime, and remember to study beta cells and the exocrine pancreas beyond autoimmunity. If different endotypes of diabetes are included in trials, new, individualized therapies can be discovered for patients in different subgroups based on genetic disposition, disease stage, and beta cell health markers. With this, embracing change and revisiting the type 1 diabetes paradigm will improve efficacy in treating new-onset disease, as well as support individualized approaches.
- In his presentation on the next steps in type 1 diabetes prevention, Dr. Haller focused on COVID-19, personalized medicine, novel trial endpoints, and the effects of new therapies’ regulatory approvals. While COVID-19 has halted many immune therapy trials, the scientific community is now restarting trials with new mitigation strategies that keep pandemic waves and the possibility of participant infection in mind. Acknowledging the heterogeneity of type 1 diabetes is required for personalized medicine. As pointed out by Dr. Haller, previous studies focused on broad risk categories based on number of antibodies and dysglycemia. He suggests new studies look at glucose tolerance, A1c, genetic risk score, pancreas volume, antibody titers, age, and Index 60, as well as incorporate early endpoints based on these markers. Along with this, Dr. Haller suggests revamped protocols, like prospective experiments and adaptive trials that answer multiple questions. Approval of new therapies may also help turn the tide toward modernizing therapies and trial designs. With teplizumab as an example, if the anti-CD3 gains FDA approval, then future studies may be required to test active comparators, against placebo, or test non-inferiority. This may also affect therapies currently under investigation, like TNF-alpha antagonist golimumab, regarding trial design and outcomes. Combination therapies also hold promise, and currently, rituximab/ abatacept is a leading candidate under development. However, study of the agent is on hold due to COVID-19. To conclude, Dr. Haller stated that durable prevention of type 1 diabetes is possible, and that researchers should look forward to developing personalized medicines.
4. Dr. Sinead Murphy on the Importance of Psychosocial Care in Adolescent Obesity
Childhood obesity is on the rise, and as described by Dr. Sinead Murphy (University College Dublin), it is a global phenomenon that requires focus on psychosocial factors to best support youth. Dr. Murphy began her presentation with sobering statistics; the average 14-year-old now weighs double what they weighed in 1948, and 15% of youth aged 10-17 are considered obese in developed countries. This presentation couldn’t be more timely, as The Robert Wood Johnson Foundation just released their 2019 State of Childhood Obesity report, which showed that 15% of American youth currently have obesity. Adolescents, defined as youth age 10-19 by the WHO, face specific challenges with puberty and brain development that complicate their experience with obesity and overweight. Dr. Murphy emphasized that obesity is also tied to neurobehavioral factors, most explicitly with food resistance related to brain development. While puberty does offer a window of opportunity with growth-spurt caloric deficits, about 90% of youth with obesity will have obesity persist into adulthood. This leads to medical, psychological, and social comorbidities, of which the latter two are key to managing obesity in up to 40% of youth. As obesity is tied to weight stigma, Dr. Murphy believes that social support and weight management groups are important components of treatment. Specifically, poor psychosocial functioning can become a treatment barrier, as many conceptualize obesity as a signal of low self-discipline, self-worth, self-esteem, and education. Dr. Murphy suggests a collaborative approach between the stakeholder, the person with obesity, and provider. With a psychosocial approach, this would take the form of an assessment, intensive treatment phase, and extensive maintenance phase. Ideally, this involves clear program eligibility, self-referrals, anthropometry, psychological assessment, a holistic approach, maximum support and weight loss goals, and proper outcome appraisals. Newer therapies for adolescents, like medications and bariatric surgery, may also be helpful as long as psychological needs are met. Overall, Dr. Murphy believes that obesity interventions for adolescents should remove blame, recognize psychosocial factors, and pair with long term support.
--by Joseph Bell, Ursula Biba, Katie Mahoney, Albert Cai, and Kelly Close