Supporting you on the front lines of pulmonary medicine.

Olympus’ new, dedicated Respiratory Division offers an array of devices designed for diagnosing and staging lung cancer and interstitial lung diseases, advancing treatments for COPD, and delivering options for visualization.
Subscribe below to receive Olympus updates.

In the respiratory arena, Olympus has been a pioneer in developing innovative solutions to advance the art of bronchoscopy. Olympus’ line of reusable diagnostic and therapeutic bronchoscopes incorporate groundbreaking technologies that improve tracheobronchial access and support enhanced visibility.
The new addition of single use, advanced procedure bronchoscopes are designed to equip physicians with the right scope, for the right patient, in the right situation.
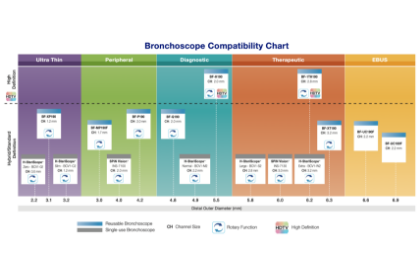
With such a diverse bronchoscopy portfolio, Olympus provides options for just about every procedure to choose between reusable or single use, giving physicians the ability to treat their patients with the best tools available for optimal clinical outcomes.
See the full line up of Olympus bronchoscopes by clicking on the Compatibility Chart image now.
(EBUS-TBNA): Proven Precision1 in Real Time

With lung cancer being the leading cause of cancer deaths worldwide,2 ways to diagnose and treat the disease at its earliest stage is more critical than ever before.
The advanced real-time technology of Olympus EBUS allows more direct, accurate, and efficient sampling, with better access to more nodal stations and smaller size lymph nodes.3 High-resolution and real-time ultrasound imaging enables direct visualization of the EBUS-TBNA needle as it penetrates the lymph node. This facilitates correct capsule-to-capsule technique, which helps to optimize sample collection.
Olympus’ respiratory portfolio can help your team manage the increased demand for more accurate and efficient methods for assessing central and peripheral regions of the lungs.
The American College of Chest Physicians Guideline Recommended Best First Test.4 The next generation of EBUS-TBNA is here.
Discover the new BF-UC190F EBUS Bronchoscope.
What You Need Is An Answer: Take Control with SPiN
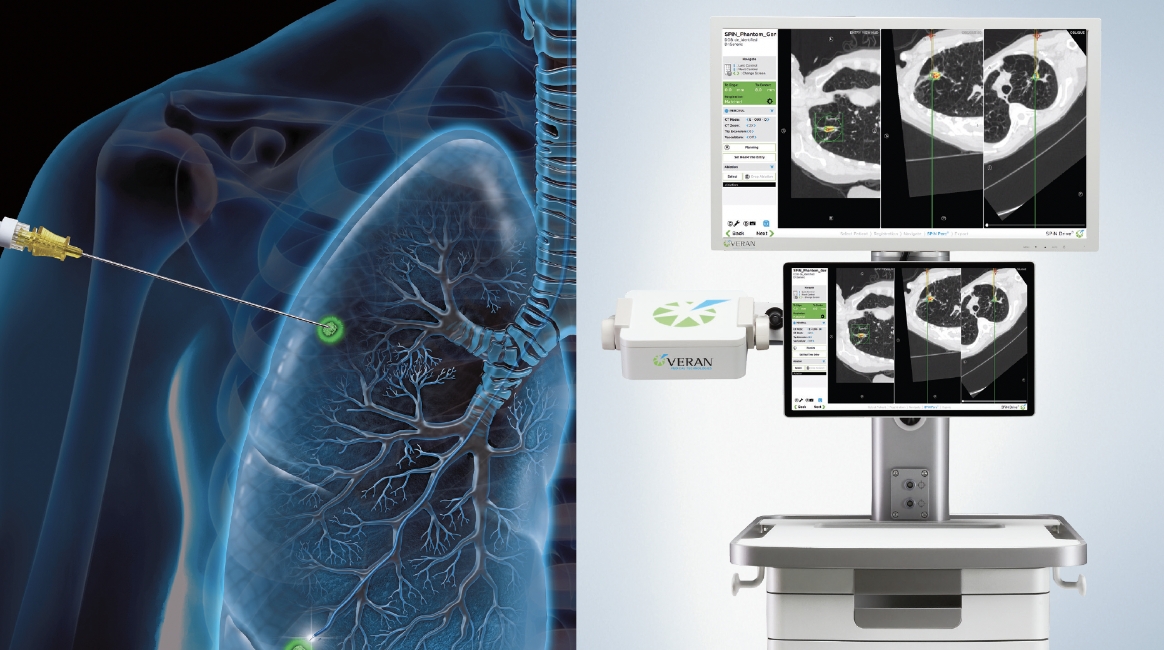
Transform the standard of care for lung cancer diagnosis and transcend procedural limitations with the SPiN Thoracic Navigation System, which puts definitive results, peace of mind, and early intervention within reach.9
The revolutionary platform is armed with a highly accurate 3D roadmap,6 respiratory gating technology, Always-On Tip Tracked™ instruments, and the flexibility to switch to a percutaneous approach with SPiN Perc™ TTNA Needle in a single procedure. The SPiN System provides precision guidance for percutaneous sampling within the bronchoscopy suite.9
46% OF NODULES MOVE A DISTANCE GREATER THAN THEIR SIZE.7
See how the SPiN System tracks this movement to help you sample at the right time.
The Right Patient. The Right Valve. The Right Outcomes.
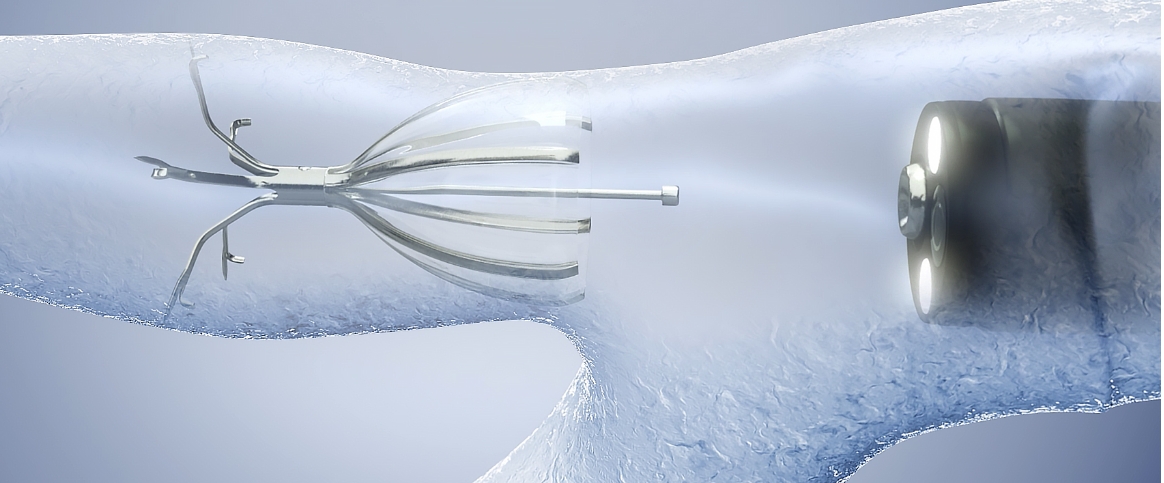
The Spiration Valve is the only valve currently on the market approved for two separate indications.10 The Spiration Valve System is an innovative endobronchial therapy that offers patients with severe emphysema a minimally invasive treatment option for lung volume reduction with a favorable risk-benefit profile.8 Patients treated with Spiration valves in clinical trials experienced improvements in breathlessness, lung function, and quality of life.7 The Spiration Valve System is also the only endobronchial valve with approval under Humanitarian Device Exemption for the management of post-surgical prolonged air leaks.11
Complete the form below to learn about our latest innovations and advancements.
1. Real-Time Endobronchial Ultrasound Guided Transbronchial Needle Aspiration for Sampling Mediastinal Lymph Nodes F J F Herth, R Eberhardt, P Vilmann, M Krasnik, A Ernst. Thorax. 2006; 61(9):795-798
2. International Agency for Research on Cancer. GLOBOCAN Lung Cancer Facts Sheet 2020.
3. Herth FJF, Ernst A, Eberhardt R, et al. Endobronchial ultrasound-guided transbronchial needle aspiration of lymph nodes in the radiologically normal mediastinum. Eur Respir J. 2006;28(5):910-914.
4. CHEST 2013; 143(5)(Suppl): 7S-37S.
5. Furukawa BS, Pastis NJ, Tanner NT, Chen A, Silvestri GA, Comparing Pulmonary Nodule Location During Electromagnetic Bronchoscopy with Predicted Location Based on Two Virtual Airway Maps at Different Phases of Respiration, CHEST (2017), doi: 10.1016/ j.chest.2017.06.004.
6. Furukawa BS, Pastis NJ, Tanner NT, Chen A, Silvestri GA. Comparing Pulmonary Nodule Location During Electromagnetic Bronchoscopy With Predicted Location on the Basis of Two Virtual Airway Maps at Different Phases of Respiration. Chest. 2018;153(1):181-186. doi:10.1016/j.chest.2017.06.004
7. Chen A, Pastis N, Furukawa B, et al. The Effect of Respiratory Motion on Pulmonary Nodule Location During Electromagnetic Navigation Bronchoscopy. CHEST. 2015;147(5):1275-1281. doi:10.1378/chest.14-1425.4
8. Criner GJ et al., 2019 Improving Lung Function in Severe Heterogenous Emphysema with the Spiration® Valve System (EMPROVE): A multi-center, Open-Label, Randomized, Controlled Trial. Am J Respir Crit Care Med, 2019. https://doi.org/10.1164/rccm.201902-0383OC
9. E L Flenaugh, K H Mohammed. Initial Experience Using 4D Electromagnetic Navigation Bronchoscopy System With Tip Tracked Instruments For Localization of Peripheral Lung Nodules. The Internet Journal of Pulmonary Medicine. 2016 Volume 18 Number 1.
10. The Spiration Valve is the only valve with both Premarket Approval for the treatment of Emphysema and Humanitarian Device Exemption for the treatment of post-surgical prolonged air leak.
11. The Spiration Valve System is also the only endobronchial valve with FDA approval under Humanitarian Device Exemption for the management of post-surgical prolonged air leaks.
12. Data on file (K170023) with Veran as of 15/May/2017
Complications from bronchoscopy are rare and most often minor, but if they occur, may include breathing difficulty, vocal cord spasm, hoarseness, slight fever, vomiting, dizziness, bronchial spasm, infection, low blood oxygen, bleeding from biopsied site, or an allergic reaction to medications. Only rarely do patients experience other more serious complications (for example, collapsed lung, respiratory failure, heart attack and/or cardiac arrhythmia).
Potential complications which may be associated with bronchoscopy and/or the Spiration™ Valve System may include, but are not limited to, pneumothorax, worsening of COPD symptoms, pneumonia, dyspnea and in rare cases, death. Prior to using the Spiration Valve System, please review the full list of prescriptive information at svs.olympusamerica.com/prescriptive-information for additional information on indications, contraindications, warnings, precautions and potential complications.
Complications from extraluminal ultrasound-guided needle aspiration may include infection, bleeding, perforation and tumor seeding. Extraluminal fine needle aspiration of cystic lesions has a higher risk of complication from infection and bleeding.
Humanitarian Device. Authorized by Federal law for use in the treatment of air leaks. The effectiveness of this device for this use has not been demonstrated.